What is Shwachman-Diamond Syndrome (SDS)
Medical and Clinical Overview

SDS is a genetic disorder. It affects many parts of the body.
You can't catch it from someone who has it. It is due to typos in the DNA, inherited from the parents.

SDS affects each person differently, with changes over time. Frequently observed symptoms include:
-
Digestive system problems and exocrine pancreatic insufficiency
➜ pain, malnutrition, slowed growth, failure to thrive, feeding issues, elevated liver enzymes
-
Immune system problems and neutropenia
➜ frequent or serious infections, urgent hospital visits
-
Problems with the bones and skeletal system
➜ hip/knee pain and possible restricted breathing, small stature
-
Brain and cognitive issues
➜ learning and behavioral challenges at home and school
-
Problems with the blood-forming system (bone marrow)
➜ bone marrow failure, low blood counts, and high risk of developing blood cancer/leukemia (e.g. MDS/AML)
➜ need for monitoring with frequent blood draws, bone marrow biopsies; some (but not all) will need a stem cell transplant.
The blood-related issues are of particular concern and can be life-threatening. About 1 in 3 SDS patients develop leukemia (AML) by age 30, with a very poor prognosis. The prevention of this complication is our focus.
Video Overview of Shwachman-Diamond Syndrome by Osmosis
More info about this video in this blog post.

In SDS, a typo in the DNA prevents the creation of enough ribosomes in the cells.
With not enough ribosomes, the cells in the body cannot make enough protein - one of the major building blocks of life. Ribosomes are huge protein complexes themselves that make all proteins in our cells by assembling amino acid chains, like hands building towers of Lego blocks. If we don’t have enough ribosomes, our cells struggle to make all the proteins - including enzymes - we need to live and thrive. No wonder that so many organ systems are affected.
Protein synthesis by Ribosomes is at the core of SDS.
Shwachman-Diamond Syndrome (SDS) is a genetic disorder in which the cells in the body cannot make enough protein - one of the main the building blocks of life. Virtually all our cellular and organ functions are carried out or depend on proteins, and so it is not surprising that many organ systems in the body are affected if the process of protein production is disrupted.

%20(1).png)
Proteins are made by ribosomes. In SDS, there are not enough of them.
Proteins are made in our cells by ribosomes, which are huge protein complexes themselves. Ribosomes make proteins by stringing together amino acids, like hands building towers of Lego blocks. The order of amino acids (Lego blocks) is specified by the genetic code in our DNA. In Shwachman-Diamond Syndrome, a genetic change in a patient’s DNA reduces the number of functional ribosomes, which in turn reduces the cells' ability to make enough protein overall. It’s like not having enough hands to meet the protein demands of the body. This can lead to an overall smaller size of the patient or some organs, but often times the impact of reduced ribosomal function is much more subtle. The impact may be invisible to the naked eye, such as stress on the bone marrow resulting in reduced blood cell production leading to fatigue and frequent infections.
Overview of Shwachman-Diamond Syndrome (SDS)
Shwachman-Diamond syndrome (SDS) is an inherited rare disease that affects many parts of the body, particularly the bone marrow, pancreas, and skeletal system. As a bone marrow failure disorder, it puts patients at high risk of life-threatening complications such as serious infections (sepsis), aplastic anemia, myelodysplastic syndrome (MDS), and acute myeloid leukemia (AML). There is no cure or targeted treatments for SDS thus far, and we need better treatment options, now!
We estimate that about 2,000-3,000 people have SDS in the United States, and a similar number in Europe, many of them un- or misdiagnosed. Exact numbers are not available, due to the difficulties with diagnosis and tracking. This number is based on an estimated incidence of SDS of 1:70,000 live births and shortened life expectancy (median in the mid-40s).
SDS Can Cause Bone Marrow & Blood Problems
The major function of bone marrow is to produce new blood cells. These include red blood cells, which carry oxygen to the body's tissues; white blood cells, which fight infection; and platelets, which are blood cell fragments that are necessary for normal blood clotting. In Shwachman-Diamond syndrome, the bone marrow malfunctions and does not make some or all types of white blood cells. A shortage of neutrophils, the most common type of white blood cell, causes a condition called neutropenia. Most people with Shwachman-Diamond syndrome have at least occasional episodes of neutropenia, which makes them more vulnerable to infections such as pneumonia, recurrent ear infections (otitis media), and skin infections. Less commonly, bone marrow abnormalities lead to a shortage of red blood cells (anemia), which causes fatigue and weakness, or a reduction in the amount of platelets (thrombocytopenia), which can result in easy bruising and abnormal bleeding.
People with Shwachman-Diamond syndrome have an increased risk of several serious complications related to their malfunctioning bone marrow. Specifically, they have a higher-than-average chance of developing myelodysplastic syndrome (MDS) and aplastic anemia, which are disorders that affect blood cell production, and a cancer of blood-forming tissue (see short video about blood cancer) known as acute myeloid leukemia (AML).
Current estimates put the cumulative incidence of MDS/AML at 20 years at 18.8%, and at 30 years at 36.1%*.
The only available curative treatment for these conditions is a hematopoietic stem cell (HSC) transplant (a.k.a. bone marrow transplant), but it is not an option for all SDS patients due to underlying health issues or the lack of a suitable donor. Crucially, once the disease has progressed to leukemia, it is too late. The prognosis of leukemia in SDS patients is extremely poor, despite the best efforts of modern transplant medicine. The current strategy to deal with this problem is to perform a HSC transplant BEFORE leukemia evolves. A central question is: when is the right time, given the high risks and toxicities associated with the procedure. It is highly recommended that SDS patients seek advice from medical centers experienced with SDS.
* GeneReviews. https://www.ncbi.nlm.nih.gov/books/NBK1756
About 1 in 5 SDS patients
will develop MDS / AML by age 20
and 1 in 3 by age 30*
AML / leukemia in
SDS patients has an extremely poor prognosis,
despite great advances in cancer treatment and transplant strategies.
SDS Can Cause Problems with the Digestive System & Pancreas
Shwachman-Diamond syndrome also affects the pancreas, which is an organ that plays an essential role in digestion. One of this organ's main functions is to produce enzymes that help break down and use the nutrients from food. In most infants with Shwachman-Diamond syndrome, the pancreas does not produce enough of these enzymes. This condition is known as pancreatic exocrine insufficiency (PEI). Infants with pancreatic insufficiency have trouble digesting food and absorbing nutrients that are needed for growth. As a result, they often have fatty, foul-smelling stools (steatorrhea); are slow to grow and gain weight (failure to thrive); and experience malnutrition (and a deficiency in fat soluble vitamins). Pancreatic insufficiency often improves with age in people with Shwachman-Diamond syndrome.
Liver problems, such as enlarged liver and elevated liver enzymes are common in SDS patients, especially in the early years. It tends to improve with age, but can again cause life threatening issues later in life. The cause and long terms effects are not well understood.
SDS Can Cause Problems with the Skeletal System & Rib Cage
Skeletal abnormalities are another common feature of Shwachman-Diamond syndrome. Many affected individuals have problems with bone formation and growth, most often affecting the hips and knees. Low bone density is also frequently associated with this condition. Some infants are born with a narrow rib cage and short ribs, which can cause life-threatening problems with breathing. The combination of skeletal abnormalities and slow growth results in short stature in most people with this disorder.
SDS Can Cause Several Additional Symptoms
The complications of this condition can affect several other parts of the body, including the heart, endocrine system (which produces hormones) - such as increased risk of diabetes, growth hormone deficiency, and adrenal insufficiency - , and eyes, teeth, and skin.
SDS Can Cause Developmental and Cognitive Challenges

Studies suggest that Shwachman-Diamond syndrome may be associated with delayed speech and the delayed development of motor skills such as sitting, standing, and walking, as well as executive function disorders and learning difficulties.
More about the Genetic Causes of SDS
As a genetic (inherited) disorder, you cannot catch SDS from someone who has SDS. It is also not something a patient can outgrow or get over with, unlike a cold.
Mutations in the SBDS gene have been identified in about 90 percent of people with the characteristic features of Shwachman-Diamond syndrome. This gene provides instructions for making a protein whose function is unknown, although it is active in cells throughout the body. Researchers suspect that the SBDS protein may play a role in processing RNA (a molecule that is a chemical cousin of DNA). This protein may also be involved in building ribosomes, which are cellular structures that process the cell's genetic instructions to create proteins. It is unclear how SBDS mutations lead to the major signs and symptoms of Shwachman-Diamond syndrome.
In cases where no SBDS mutation is found, the cause of this disorder is unknown.
There are slightly more males diagnosed than females, and SDS is found in all ethnic groups.
Over 90% of SDS is due to mutations in a gene called SBDS. There are a few other genes associated with SDS or SDS-like syndromes, but they account for only a handful of patients. The genetic cause for the remaining <10% of patients is still unknown. See details at https://www.ncbi.nlm.nih.gov/books/NBK1756/.
How is SDS Inherited?
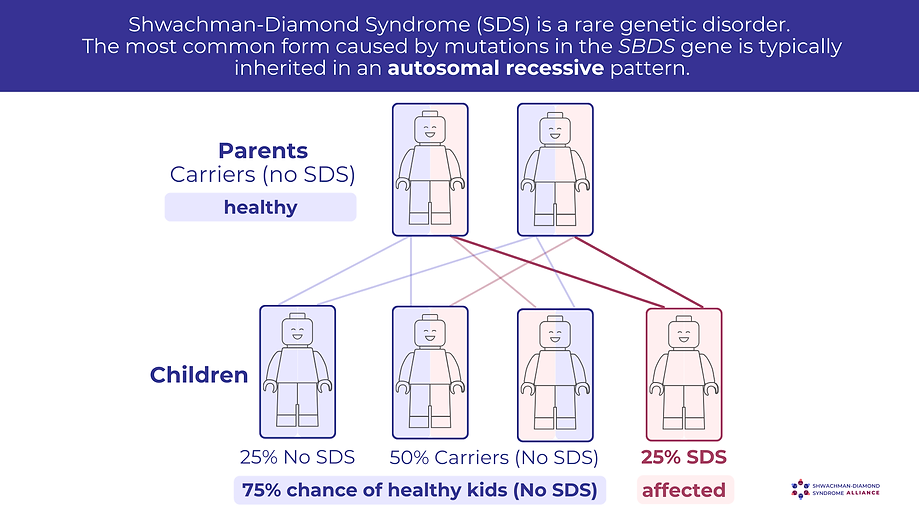
SDS caused by SBDS gene mutations is inherited in an autosomal recessive pattern, which means both copies of the gene in each cell need to have a mutation in order to cause SDS. Typically, the parents of an individual with an autosomal recessive condition each carry one copy of the mutated gene (they are carriers), but they do not show signs or symptoms of the condition. Parents who are carriers of SBDS mutations have a 25% (1 in 4) chance of having a child with SDS, a 50% chance of their children being carriers (no symptoms), and a 25% chance of their children being unaffected (free of the SBDS mutations).
There are also reports of some cases in which a patient inherited only one mutated copy of the SBDS gene from one parent, and acquired a new spontaneous mutation in the other copy during embryonic development.
More Information about SDS on GARD by NIH/NCATS:
Further reading:
Disclaimer: The information provided on this website should NOT be used as a substitute for seeking professional medical diagnosis, treatment or care. You should not rely on any information in these pages to replace consultations with qualified health professionals.
Most of the text and several illustrations on this page are courtesy of the US National Library of Medicine > Genetics Home Reference. We added updates and additional details from current scientific and medical publications. Reviewed by out Medical and Scientific Advisory Board.








